The Ministry of Education, Culture, Sports, Science and Technology has developed a research and development strategy for reducing greenhouse gases. The Science and Technology Promotion Agency (JST) is promoting the development of “Advanced Carbonization Technology (ALCA)†under the guidance of this strategy. In February 2016, the project was held. A technical briefing on "New Generation Battery", one of the development fields. Northeastern University and Kansai University introduced the development of new basic technologies aimed at achieving lithium-sulfur (LIS) batteries.
Actively developed as a powerful candidate for "post-lithium-ion batteries" is the LIS battery. This briefing session introduces a number of basic technologies being developed to implement LIS batteries. One of them is the solid electrolyte developed by the research and development team led by Prof. Yan Mao and Prof. Yu, who is a professor at the Institute of Advanced Studies in Atomic and Molecular Materials Science, Tohoku University, Japan. The use of complex hydrides in its electrolytes is highly anticipated for use on LIS cells.
The LIS battery is a battery in which a positive electrode material is made of sulfur and a negative electrode material is made of metal lithium. The theoretical capacity density of sulfur as a positive electrode material is about 1670 mAh/g, which is more than 6 times that of a ternary material commonly used for a positive electrode material of a lithium ion battery. In addition, the theoretical capacity density of metallic lithium as a negative electrode material is 3861 mAh/g, which is about 10 times that of a negative electrode material carbon (372 mAh/g) which is commonly used for lithium ion batteries. The energy density is expected to increase significantly compared to current lithium-ion batteries.
However, the problem with the LIS battery is that if the electrolyte uses an organic electrolyte commonly used for lithium ion batteries, the battery capacity will be significantly reduced with the charge and discharge cycle. The intermediate compound of sulfur and lithium which is formed during the charge and discharge reaction of the battery is dissolved in the electrolytic solution, and a reaction occurs on the negative electrode side, so that the amount of sulfur used for charge and discharge is greatly reduced.
Change electrolyte or carbon material
In this regard, one of the countermeasures considered is to prevent sulfur elution by using a solid electrolyte that is more stable than liquid. Northeastern University's research and development team is developing complex hydrides that can be used in such solid electrolytes.
The R&D team focused on complex hydrides because of the high stability of the materials used in batteries. Yu said that "sulfide and oxide have been widely studied as solid electrolytes. Although there are very high ion conductivity and can be used in battery types, there are not many types of stability required for battery operation." The complex hydride refers to a metal cation M (Li+, Na+, Mg2+, etc.) and a complex anion (M'H)n [(BH4)-, (NH2)-, (AlH4)-, (AlH6)3-, etc. The M(M'H)n substance formed. It is not easily thermally decomposed at a high temperature of 150 ° C, and a light element can be used as a constituent element, and a precise electrolyte can be produced by uniaxial pressing at room temperature. However, the ionic conductivity is low and the operating temperature is high.
For example, current electrolyte ionic conductivity is 10-2 S/cm or more (room temperature). Lithium borohydride (LiBH4), one of the complex hydrides, has an ionic conductivity of 2 & TImes at a temperature of 390 K (about 120 ° C); 10-3 S/cm or more, and about 10-7 S/cm at room temperature (Fig. 1 ,2). The research and development team changed the ionic conductivity at room temperature to about 10-5 to 10-4 S/cm by replacing a part of BH4 ion [(BH4)-] with iodide. However, Yu said at all, "To achieve the same level of energy density and output density as current lithium-ion batteries, it needs to be increased to about 10-3 S/cm." In addition to LiBH4, the R&D team is exploring other complex hydrides. Li2B12H12 (about 10-4 S/cm at 60 ° C) and compounds of LiNH 2 and LiBH 4 are also candidates.
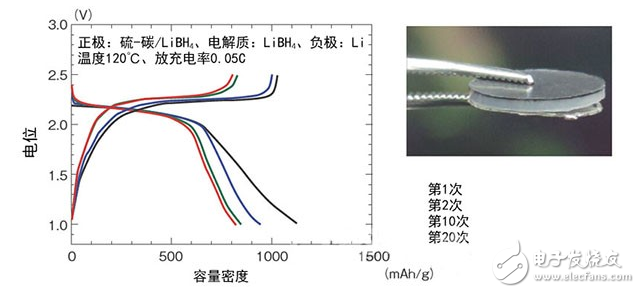
Figure 1: Performance evaluation of a prototype block solid lithium-sulfur battery
Northeastern University professor Ningmao and others' research and development team developed. The positive electrode is made of sulfur and has a capacity density of up to 800 mAh/g (20th time).
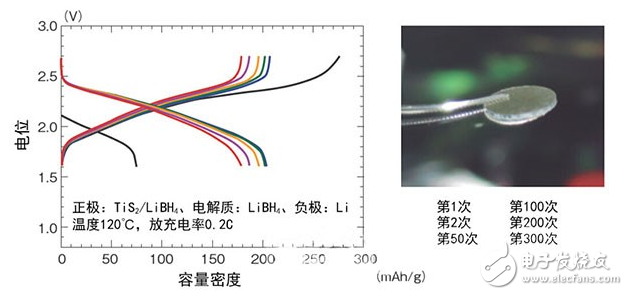
Figure 2: Performance evaluation of a prototype block solid all-solid TIS2/Li battery
Northeastern University professor Ningmao and others' research and development team developed. The positive electrode is TIS2, and it can be repeatedly charged and discharged more than 300 times at 0.2C.
In fact, the R&D team used the high heat resistance of the complex hydride to jointly develop the basic technology of a lithium ion battery that can be used in a higher temperature engine compartment with Hitachi. This technique is applied to a lithium ion battery and maintains a theoretical capacity of 90% at a high temperature of 150 ° C (Fig. 3).
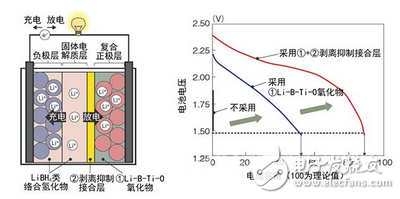
Figure 3: Basic technology for achieving high heat-resistant lithium-ion batteries
The research and development team of Tohoku University's professors was developed jointly with Hitachi. The left is the battery structure. Right is the relationship between battery voltage and battery capacity. The new technology (1+2) ensures a battery capacity of 90% of the theoretical capacity.
The main point of this is that by sandwiching the mixture layer of the complex hydride LiNH2 and LiBH4 between the positive electrode layer and the solid electrolyte layer, the peeling phenomenon which occurs with the volume change accompanying charge and discharge between the two is prevented. Further, Li-BTI-O (lithium-boron-titanium-oxygen)-based oxide is used as a binder of the ternary active material used as the positive electrode material, and the decomposition reaction of the positive electrode material with LiBH4 is prevented.
In addition, in order to prevent the sulfur dissolution of the LIS battery, the research and development team led by Professor Ishikawa Masahiro and Associate Professor Yamagata Yaji of the Kansai University's Department of Chemical and Bioengineering developed a method to change the carbon material that adsorbs sulfur using a positive electrode instead of an electrolyte. According to Yamagata, "If a carbon material having pores having a diameter of 1 nm or less is used, sulfur flowing into the pores is not easily released", thereby preventing sulfur from decreasing as the charge and discharge cycle occurs.
In addition to this carbon material, the R&D team increased the output power of the LIS battery by using sodium alginate for the sulfur positive electrode binder. One of sodium alginate, magnesium alginate, is used as a binder. The sulfur-activated carbon composite active material and the conductive auxiliary acetylene black are solidified together by using magnesium alginate to prepare a positive electrode material. The negative electrode material was made of lithium metal and the electrolyte was prepared by using an ionic liquid (a low melting point salt composed only of a cation and an anion plasma). After a charge and discharge cycle of 0.5 C for 15 times, a high capacity of about 900 mAh/g was maintained. Other ionic liquids were used as the electrolyte, and there was a type in which a high capacity of about 900 mAh/g was maintained after 70 cycles of charge and discharge, and a type that could operate as a battery at 2.0C.
Sweeper Battery,Lifepo4 Power Pack For E-Sweeper,Swivel Sweeper Lithium Ion Battery,Lifepo4 Battery For Floor Sweeper
JIANGMEN RONDA LITHIUM BATTERY CO., LTD. , https://www.ronda-battery.com