The electrolyte is an ionic conductor that conducts electricity between the positive and negative electrodes of the battery. It is composed of electrolyte lithium salt, high-purity organic solvent and necessary additives in a certain ratio. The energy density, power density and wide temperature of the battery are Applications, cycle life, safety performance and other aspects play a vital role.

The lithium battery is composed of a casing, a positive electrode, a negative electrode, an electrolyte and a separator, and the electrode material is undoubtedly the focus of attention and research. But at the same time, the electrolyte is also a non-negligible aspect. After all, the electrolyte that accounts for 15% of the battery cost does play a vital role in the energy density, power density, wide temperature application, cycle life, and safety performance of the battery. character of.
The electrolyte is an ionic conductor that conducts electricity between the positive and negative electrodes of the battery, and is prepared by a predetermined ratio of raw materials such as an electrolyte lithium salt, a high-purity organic solvent, and necessary additives.
As people's requirements for the performance of new energy vehicle power batteries continue to increase, lithium batteries continue to be iteratively upgraded, and it is an indisputable fact that they have begun to enter the high-nickel three-yuan era. However, if the electrolyte cannot be upgraded with the battery material, the high-nickel ternary system is difficult to achieve its original design.

The increasing proportion of nickel in the positive electrode material and the use of the silicon carbon negative electrode bring new troubles to the development and production of the electrolyte.
As the energy density of the power battery increases, the voltage will also increase. The higher the voltage, the stronger the decomposition ability of the electrolyte. For the high-nickel ternary system, the electrolyte company has specifically tested the leakage current (ie, the current flowing through the insulator) and the transition of the transition metal ions. The test results show that the nickel content in the positive electrode material of the power battery increases, and the dissolution of the transition metal ions increases. The eluted transition metal ions destroy the SEI film on the surface of the negative electrode after the negative electrode is reduced and precipitated. In addition, increasing the voltage also significantly increases the leakage current. In this way, the storage performance and cycle performance of the power battery in a high temperature environment are affected, and the increase in the nickel content in the material also leads to a decrease in the safety performance of the power battery.
These problems arising from the use of high-nickel materials in combination with electrolytes are complicated to solve and have high technical thresholds. If the company does not have sufficient research and development capabilities, it is difficult to make electrolyte products that match high nickel materials.
1. High specific energy electrolyte
The pursuit of high specific energy is currently the largest research direction of lithium-ion batteries, especially when mobile devices occupy more and more people's lives, and the battery life is the most critical performance of batteries.
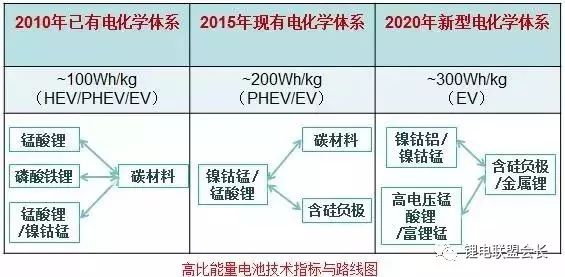
Image source: Beijing Institute of Chemical Reagents
As shown in the figure, the development of high-energy density batteries in the future must be high-voltage positive electrodes and silicon negative electrodes. The negative silicon has a large gram capacity and has attracted attention. However, due to its own swelling effect, it has not been applied. In recent years, the research direction has been changed to a silicon carbon negative electrode, which has a relatively high gram capacity and a small volume change. The film forming additive has different cycling effects in the silicon carbon negative electrode.
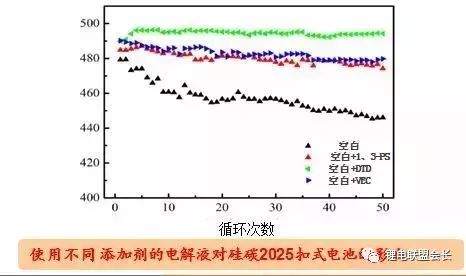
Image source: Beijing Institute of Chemical Reagents
2, high power electrolyte
At present, it is difficult to achieve high-rate continuous discharge of commercial lithium-ion batteries. The main reason is that the battery ear is extremely hot, and the internal resistance of the battery is too high, which is prone to thermal runaway. Therefore, it is required that the electrolyte can suppress the temperature rise of the battery too fast while maintaining high conductivity. For power batteries, achieving fast charging is also an important direction for the development of electrolytes.

High-power batteries not only require high solid-phase diffusion, nano-imprinting, short ion migration path, thickness of control sheet and compaction, but also put forward higher requirements for electrolyte: 1. High dissociation electrolyte Salt; 2, solvent compounding - lower viscosity; 3, interface control - lower membrane impedance.
3, wide temperature electrolyte
When the battery is at a high temperature, the decomposition of the electrolyte itself and the side reaction of the material and the electrolyte are prone to increase; at low temperatures, electrolyte salt precipitation may occur and the impedance of the negative SEI film may be multiplied. The so-called wide temperature electrolyte is to make the battery have a wider working environment. The figure below shows the boiling point comparison chart and solidification comparison chart of various solvents.
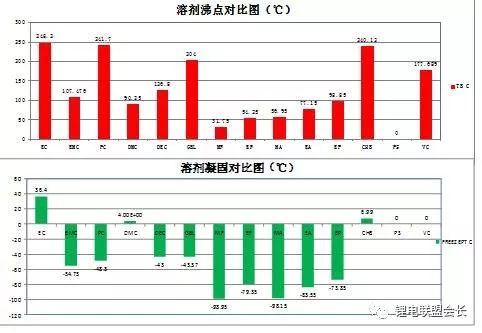
Image source: Beijing Institute of Chemical Reagents
4, safety electrolyte
The safety of the battery is mainly reflected in the burning or even the explosion. First, the battery itself is flammable. Therefore, when the battery is overcharged, overdischarged, or short-circuited, when the external acupuncture and extrusion are received, when the outside temperature is too high, Both can cause a security incident. Therefore, flame retardancy is a major direction in the study of safe electrolytes.
The flame retardant function is obtained by adding a flame retardant additive to a conventional electrolyte. Generally, a phosphorus-based or halogen-based flame retardant is used, and the flame-retardant additive is required to be reasonably priced and does not impair the electrolyte performance. In addition, the use of room temperature ionic liquids as electrolytes has also entered the research stage, and the use of flammable organic solvents in batteries will be completely eliminated. And the ionic liquid has the characteristics of extremely low vapor pressure, good thermal stability/chemical stability, and non-flammability, which will greatly improve the safety of the lithium ion battery.
5, long circulating electrolyte
Due to the current technical difficulties in the recovery of lithium batteries, especially the recovery of power batteries, improving battery life is one way to alleviate this situation.
There are two main research ideas for long-circulating electrolytes. One is the stability of the electrolyte, including thermal stability, chemical stability, and voltage stability. Second, the stability with other materials requires stable film formation with the electrode. No oxidation with the diaphragm, no corrosion with the current collector.
Dc Solar Pump,Solar Centrifugal Pump,Surface Centrifugal Pump ,Dc Solar Water Pumps
Wuxi Doton Power , http://www.dotonpower.com